Human Health
The Tripping Point
In recent decades, humanity has made great progress in curing diseases of the body, but little progress has been made in treating diseases of the mind.
Mental healthcare, meanwhile, has become a leading public health crisis in America. Nearly 50% of Americans will experience mental illness during their lifetimes. A CDC survey released in 2020 reported that 13.2% of U.S. adults were using antidepressants and we believe that number has only increased since the pandemic. We estimate over 38M Americans are currently on antidepressants, and 20M Americans are on anti-anxiety medications. Yet current treatments are far from sufficient. SSRIs, including drugs like Prozac and Zoloft, are one of the most popular interventions for depression, but have a 30% failure rate. We clearly need better, longer lasting alternatives.
Some of those alternatives, it turns out, have been with us all along.
Research into the power of psychedelic medicine to treat mental health issues including depression, anxiety, and PTSD is booming. According to the U.S. National Library of Medicine, there are hundreds of trials underway or actively recruiting at universities around the country including Johns Hopkins, Stanford, and Harvard, and at institutions like the U.S. Department for Veterans Affairs. Many of these trials of drugs including ketamine, MDMA, and psilocybin have produced remarkable early results.
At Obvious, we believe there are venture-backable companies in this category that can deliver transformative results for human health.We are investing in companies who are following the scientific pathway for approval and we envision a near future where these breakthrough medicines are FDA-approved and accessible to patients through their healthcare providers.
Back to the future
Also known as hallucinogens, psychedelics are a class of psychoactive substances that alter perception and mood, and promote new neural pathways in the brain. Some psychedelics occur in nature—psilocybin, mescaline, and DMT—while others are created in labs, including MDMA, LSD, and ketamine.
Psychedelics for human health isn’t a new idea. Their use has been part of some indigenous cultures for centuries. Scientific research into their impact goes back to the 1950s and 1960s, when more than 1,000 articles were published about the use of psychedelics for psychiatric treatment. However, as the recreational use of psychedelics spread among the counterculture, the American government stepped in and banned them under the Controlled Substances Act of 1970. As designated Schedule 1 substances, they were classified as having no accepted medical use. The government also created a public narrative that drugs like LSD can cause you to lose your mind and hurt yourself or others (these narratives have since been scientifically disproven).
For many years, psychedelic research languished, essentially locked-up by the FDA and the DEA. With one exception: in 1970 the FDA approved ketamine for use as an anesthetic and sedative during the Vietnam War. This started the clock on what is now a multi-decade data record of safety for medical use of ketamine in humans.
Then, in 1990, the FDA began allowing research on the naturally occurring psychedelic DMT. In 1999, research on psilocybin was granted. And in 2017, after many years of research and trials conducted by an organization called the Multidisciplinary Association for Psychedelic Studies (MAPS), the FDA granted Breakthrough Therapy Designation to MDMA. This summer, the Biden Administration announced it is planning full approval of MDMA for prescription use for PTSD sometime within the next 24 months.
In 2019, the FDA approved a ketamine-based antidepressant called Spravato for treatment-resistant depression. Spravato, a variant of ketamine called esketamine, is prescribed in conjunction with an oral antidepressant for adults who have tried other antidepressant medicines but have not benefited from them. Today physicians are able to prescribe both generic ketamine (off-label) and Spravato (on-label) for psychiatric use.
Meanwhile, the floodgates have opened on clinical trials of other psychedelics. Headlines about the promising results of these trials for the treatment of everything from addiction to Alzheimers are becoming commonplace. States such as Oregon, and cities such as Denver, Oakland, and Ann Arbor, have decriminalized the use of certain psychedelics, and a number of psychoactive companies have gone public on the Toronto Stock Exchange.
A cultural and market shift is underway.
The next phase
Even though some psychedelics have been used by humans for centuries and we know they are safe, there’s a non-trivial cost to bringing them to market as medicine. That’s where venture capital comes in. The cost of drug discovery, from research to manufacturing, is enormous and can exceed $1B in expenditures per new drug developed. Venture capital closes the gap between university research and commercialization.
There is also a need for ethics in this emerging space. We need protocols for how we care for patients with these medicines. The North Star Pledge is one example. It was written to create shared values for the psychedelic field.
Additionally, there is a need to honor the ancient knowledge that much of our understanding of plant-based psychedelics is built on. We are standing on the shoulders of giants, the indigenous peoples who have been using plant medicine as part of their cultural and spiritual practices for centuries. Several startups in psychedelic medicine are inventing new business models that include indigenous peoples as stakeholders.
Stacked with discovery and ethics, we also need affordability and access for these powerful psychedelic medicines. Enthea is a new non-profit benefit plan administrator on a mission to expedite health insurance coverage for psychedelic therapies. Ethnea works with health insurance companies, and self-insured employers, to add policies and coverage for psychedelic-assisted therapy. This is critical work on the journey towards safe, affordable access to psychedelic therapies for all who can benefit.
Investing in psychedelics
At Obvious, our investment focus is on the subset of the psychedelics universe that has the longest safety record of human use. Our big four are ketamine, psilocybin, MDMA, and DMT.
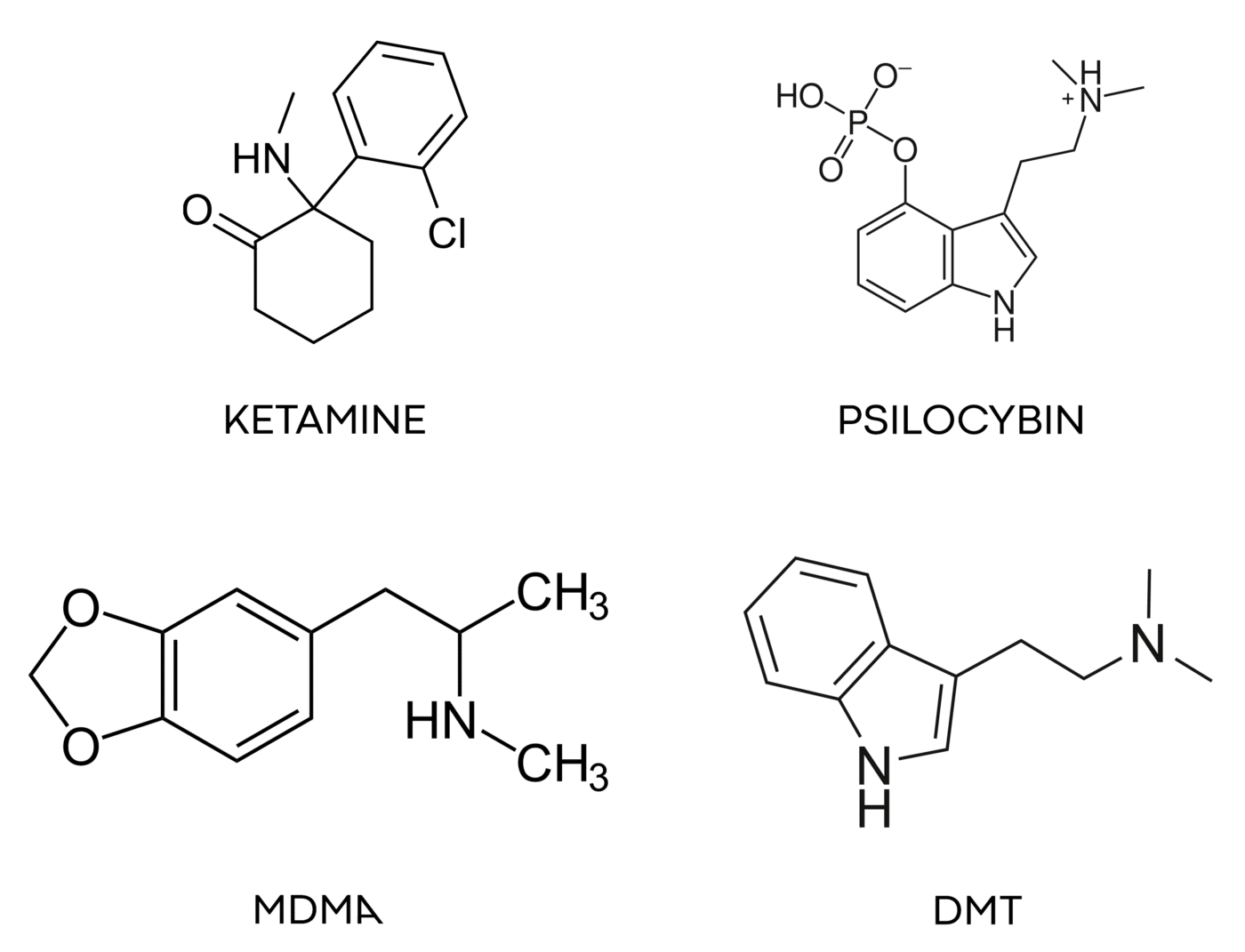
Ketamine can be prescribed today and is showing great promise for a range of mental health issues. Psilocybin and MDMA are the next two molecules likely to gain regulatory approval, with many clinical trials well underway. The DMT family has many molecules within it, and a rich history of spiritual and cultural use by humans for millennia. Because of the deep knowledge and safety record among these four families, we believe they have a high probability of becoming approved medicines.
Our first investment in psychedelic medicine is MycoMedica Life Sciences, a Public Benefit Corporation developing fungi-based therapeutic drugs. MycoMedica is founded by Paul Stamets, the world-renowned mycologist and star of an episode of Netflix’s recent docuseries “How to Change Your Mind.”
MycoMedica’s breakthrough innovation is the Stamets Stack, which combines three ingredients not found together in nature to unlock the potential effectiveness of psilocybin in the prevention and treatment of psychiatric and neurological disorders. The company’s initial research is focused on microdosing, which also allows for greater patient affordability and accessibility.
Our second investment is Nue Life, a digital delivery platform for drug-assisted therapy, beginning with at-home ketamine treatments. The Nue Life team have created a therapeutic ecosystem of ketamine therapy, an interactive companion app, and virtual aftercare programs designed to help patients improve their mental health and wellness. They successfully treated 3,000 patients and facilitated over 20,000 ketamine experiences in their first year of operations, while measuring the data on all patient outcomes.
A recent study conducted by Nue Life, to be published in Frontiers of Psychology, found that almost 66% of patients suffering from treatment-resistant depression reported at least a 50% decrease in their symptoms after completing the Nue Life treatment program. Nue Life’s next focus is how to make these incredible improvements more durable and longer lasting for their patients.
These soon-to-be former Schedule 1 drugs like MDMA and psilocybin are already having profound breakthrough benefits for human health. As they move through the FDA approval process, we hope to get these medicines to millions of customers in an affordable and accessible way.
If you’re an entrepreneur building a world positive psychedelic startup, we’d love to hear from you. Drop us a line at: psychmed@obviousventures.com
Obvious Ideas